Stem Cells:上海交大杨黄恬研究组揭示组蛋白去甲基化酶PHF8在调控
2月11日,国际学术期刊Stem Cells在线发表了中国科学院上海生命科学研究院/上海交通大学医学院健康科学研究所杨黄恬研究组题为“PHF8 regulates mesodermal and cardiac differentiation of embryonic stem cells through mediating the histone demethylation of pmaip1”的最新研究成果,该研究报告了组蛋白去甲基化酶PHF8调节中胚层及心肌细胞分化的新作用,并揭示了其作用机制。博士研究生汤燕、洪雅贞为论文共同第一作者,杨黄恬研究员为论文第一作者。
胚胎干细胞具有在体内外分化为三胚层及其衍生细胞的能力。因此,对体外胚胎干细胞分化机制的研究将有助于理解哺乳动物发育过程中的调控机制。例如类似于体内胚胎及胚泡形成的早期胚胎干细胞体外分化,其分化过程中伴随着细胞增殖能力的下降和凋亡的上升。此前杨黄恬研究团队和其它实验室便发现钙离子通道三磷酸肌醇受体IP3R3调控的钙离子释放和MAPK通路激活介导的凋亡参与调控了干细胞定向分化的进程,部分解释了凋亡对早期发育中细胞谱系命运决定的作用和调控方式。然而,相比于已被广泛揭示的受信号通路调控的凋亡机制,对表观遗传修饰尤其是组蛋白去甲基化酶(KDMs)在胚胎干细胞分化过程中凋亡的调控作用,及该调控在胚胎干细胞谱系分化中的意义了解非常有限。phf8(plant homeo domain finger protein 8)是位于X染色体p11.22上的基因,属于组蛋白去甲基化酶JmjC(Jumonji C)家族。研究发现在P19细胞中敲减phf8会抑制其神经分化能力,而在斑马鱼中该基因的缺失导致神经分化异常。然而,对PHF8在胚胎干细胞分化过程中的作用及机制知之甚少。
博士研究生汤燕、洪雅贞等在杨黄恬研究员的指导下发现组蛋白去甲基化酶PHF8通过调控凋亡蛋白PMAIP1,从而影响胚胎干细胞向中胚层及心肌细胞的分化。在机制方面,胚胎干细胞向中胚层及心肌细胞分化过程中,PHF8结合到凋亡基因pmaip1的启动区上并移除其上的抑制性标记H3K9me2从而促进pmaip1的转录表达,上调中胚层和心血管前体细胞的凋亡,进而削弱胚胎干细胞向中胚层及随后的心肌细胞的分化,但并不影响早期外胚层和内胚层分化。研究结果揭示了PHF8在ESCs在中胚层及心肌细胞分化过程中的重要作用及其经由细胞凋亡来调控细胞分化的新机制。该研究发现不仅扩展了对KDMs在ESCs分化及凋亡中作用的认识,并进一步证实了分化与凋亡之间的直接关系。
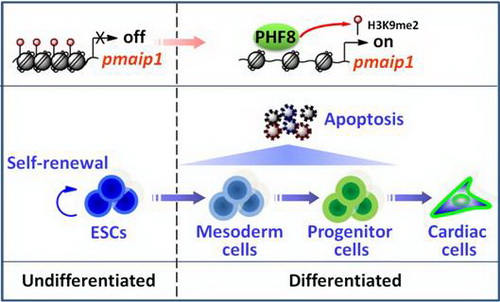
图:组蛋白去甲基化酶PHF8通过调控凋亡基因pmaip1,促进细胞凋亡从而抑制胚胎干细胞向中胚层及心肌细胞分化
原文链接:
PHF8 regulates mesodermal and cardiac differentiation of embryonic stem Cells through mediating the histone demethylation of pmaip1
原文摘要:
Histone demethylases have emerged as key regulators of biological processes. The H3K9me2 demethylase PHF8 (plant homeo domain finger protein 8), for example, is involved in neuronal differentiation, but its potential function in the differentiation of embryonic stem cells (ESCs) to cardiomyocytes is poorly understood. Here we explored the role of PHF8 during mesodermal and cardiac lineage commitment of mouse ESCs. Using a phf8 knockout (ph8-/Y) model, we found that deletion of phf8 in ESCs did not affect self-renewal, proliferation or early ectodermal/endodermal differentiation, but it did promote the mesodermal lineage commitment with the enhanced cardiomyocyte differentiation. The effects were accompanied by a reduction in apoptosis through a caspase 3-independent pathway during early ESC differentiation, without significant differences between differentiating wide-type (ph8+/Y) and ph8-/Y ESCs in cell cycle progression or proliferation. Functionally, PHF8 promoted the loss of a repressive mark H3K9me2 from the transcription start site of a pro-apoptotic gene pmaip1 and activated its transcription. Furthermore, knockdown of pmaip1 mimicked the phenotype of ph8-/Y by showing the decreased apoptosis during early differentiation of ESCs and promoted mesodermal and cardiac commitment, while overexpression of pmaip1 or phf8 rescued the phenotype of ph8-/Y ESCs by increasing the apoptosis and weakening the mesodermal and cardiac differentiation. These results reveal that the histone demethylase PHF8 regulates mesodermal lineage and cell fate decisions in differentiating mouse ESCs through epigenetic control of the gene critical to programmed cell death pathways.
作者:杨黄恬

