Plant Cell:中科院植生生态所王永飞研究组发现ABA信号途径与光信
2016年3月22日,国际植物学著名期刊《The plant Cell》杂志在线发表了中国科学院上海生命科学研究院植生生态所王永飞研究组题为“S-type Anion Channels SLAC1 and SLAH3 Function as Essential Negative Regulators of Inward K+ Channels and Stomatal Opening in Arabidopsis”的研究论文。该研究发现了一个ABA信号途径通过蛋白互作抑制光信号途径,并因此抑制植物气孔开放的新机制。博士研究生张安、任慧敏等人为论文共同第一作者,王永飞教授为论文通讯作者。
干旱胁迫和ABA信号诱导气孔关闭,从而减少水分散失,提升植物的耐旱能力。而光照则可以诱导气孔开放,便于植物吸收CO2和释放氧气,同时散失大量水分,促进植物的生长发育。但光照诱导气孔开放必须是在没有干旱胁迫和ABA信号刺激的前提下才可以实现,否则光信号无法诱导气孔开放,即干旱/ABA信号途径可以有效抑制光信号途径及其诱导的气孔开放。但多年来,干旱信号途径抑制光信号途径从而进一步抑制光诱导的气孔开放运动的信号传递机理并不清楚。本研究发现,干旱诱导拟南芥气孔保卫细胞中大量质膜慢阴离子通道(S-type anion channels)SLAC1和SLAH3的蛋白积累。SLAC1和SLAH3一方面作为质膜慢阴离子通道介导阴离子跨质膜外流,同时大量积累的SLAC1和SLAH3蛋白还通过蛋白互作的方式,强力抑制气孔保卫细胞质膜内向K+通道,以此有效抑制光诱导的气孔开放。众所周知,SLAC1和SLAH3是ABA信号途径中的关键组份,而质膜内向K+通道则是光信号途径的关键组份。因此,本研究揭示了一个干旱/ABA和光信号途径互作,并共同参与气孔运动调控的新机制。
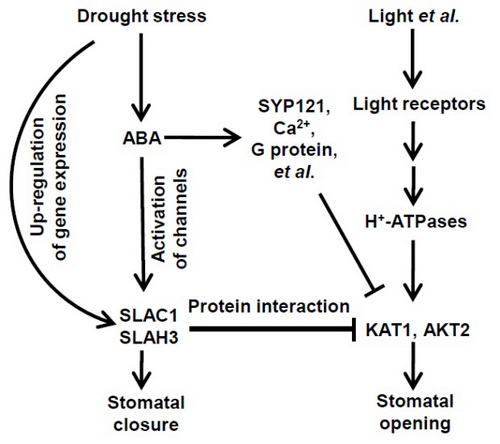
图:ABA信号途径与光信号途径互作新机制
原文链接:
S-type Anion Channels SLAC1 and SLAH3 function as Essential Negative Regulators of Inward K+ Channels and Stomatal Opening in Arabidopsis
原文摘要:
Drought stress induces stomatal closure and inhibits stomatal opening simultaneously. However, the underlying molecular mechanism is still largely unknown. Here we show that S-type anion channels SLAC1 and SLAH3 mainly inhibit inward K+ (K+in) channel KAT1 by protein-protein interaction, and consequently prevent stomatal opening in Arabidopsis. Voltage-clamp results demonstrated that SLAC1 inhibited KAT1 dramatically, but did not inhibit KAT2. SLAH3 inhibited KAT1 to a weaker degree relative to SLAC1. Both the N terminus and the C terminuses of SLAC1 inhibited KAT1, but the inhibition by the N terminus was stronger. The C terminus was essential for the inhibition of KAT1 by SLAC1. Furthermore, drought stress strongly up-regulated the expression of SLAC1 and SLAH3 in Arabidopsis guard cells, and the over-expression of wild type and truncated SLAC1 dramatically impaired K+in currents of guard cells and light-induced stomatal opening. Additionally, the inhibition of KAT1 by SLAC1 and KC1 only partially overlapped, suggesting that SLAC1 and KC1 inhibited K+in channels using different molecular mechanisms. Taken together, we discovered a novel regulatory mechanism for stomatal movement, in which singling pathways for stomatal closure and opening are directly coupled together by protein-protein interaction between SLAC1/SLAH3 and KAT1 in Arabidopsis.
作者:王永飞

