Blood:北京大学黄晓军研究组揭示PML-RARA “突变热点区”导致白血
近日,国际著名血液学顶级期刊《Blood》在线发表了北京大学人民医院血液病研究所黄晓军教授的一篇研究论文,研究组揭示PML-RARA “突变热点区”导致白血病耐药机制。北京大学人民医院血液病研究所刘江莹助理研究员和主鸿鹄副主任医师为共同第一作者,北大-清华生命科学联合中心研究员、北京大学人民医院血液病研究所黄晓军教授为通讯作者。
急性早幼粒细胞白血病(APL)是一种起病凶险的恶性血液病,单纯化疗后患者复发率高,总体生存较差。黄晓军课题组证实口服或静脉砷剂取得相似的疗效和安全性,联合维甲酸根治率达90%以上;随后针对非高危APL患者的前瞻临床试验实现了“不输液、不化疗、不住院“治愈白血病;这些成果近2年发表于医学顶级期刊《New England Journal of Medicine》(新英格兰医学杂志)、肿瘤学顶级期刊《Journal of Clinical Oncology》(临床肿瘤学杂志)。 同期研究表明,即使选用了这种治疗,仍有部分高危患者复发,再用砷剂治疗效果较差。因此,砷剂耐药机制已成为APL相关研究领域的关键科学问题。课题组前期发现难治/复发APL患者PML-RARA基因中存在一个“突变热点区”包括4个新的PML-RARA突变位点: A216T,S214L,L217F和S220G,可能介导耐药,成果也发表于《新英格兰医学杂志》。
在前述工作基础上,本研究利用一系列分子生物学和细胞生物学研究方法,以人宫颈癌细胞系(HeLa)、人髓性白血病细胞系(U937)以及CD34阳性正常造血干细胞为模型,阐明含有A216V,A216T或S214L突变的PML-RARA融合蛋白在常规剂量的砷剂处理后未发生降解,而在同样实验条件下,含有L217F或S220G的融合蛋白则发生显著降解, 提示PML-RARA融合基因点突变的生物学功能存在差异, 进而导致砷剂对PML-RARA融合蛋白的命运产生不同影响。这一发现在临床难治/复发的APL患者中得到进一步验证。本研究还发现,在体外实验中增加细胞内砷剂浓度、或者联合应用常规浓度砷剂与维甲酸可以克服PML点突变引起的砷剂耐药。该研究进一步完善了对APL患者砷剂耐药机制的认识,为下一步克服耐药研究提供了靶点。同时,这一成果将有助于在临床治疗APL过程中对患者进行砷剂耐药监测,实现APL的分层和个性化治疗,从而提高临床治愈率,减少复发。
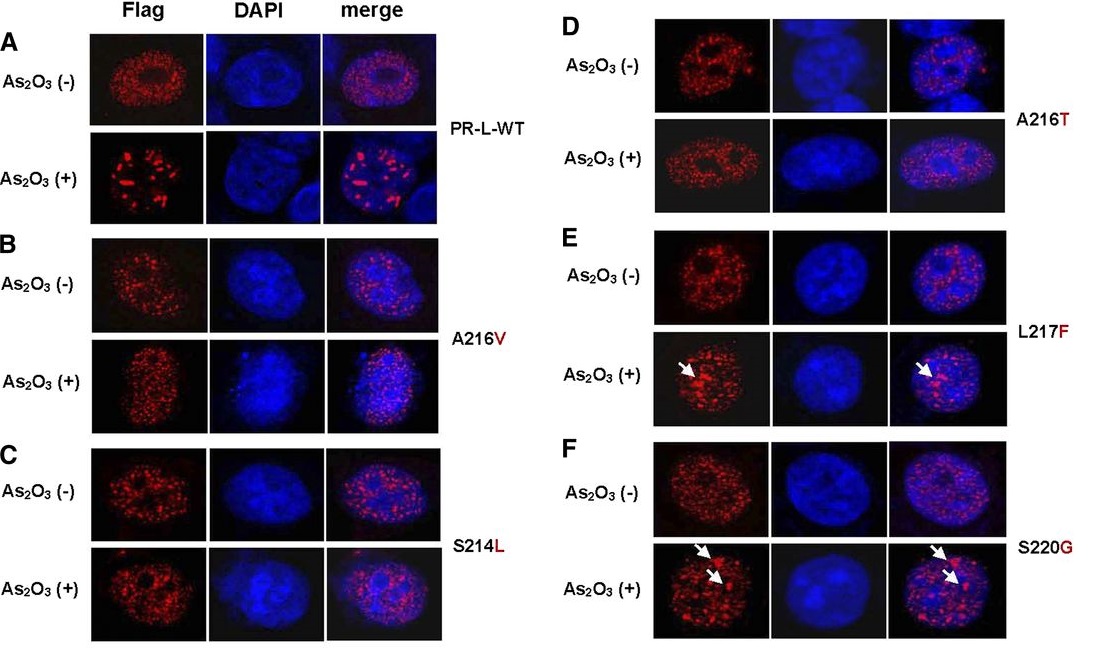
图:PML-RARA “突变热点区”导致白血病耐药机制
原文链接:
Varying responses of PML-RARA with different genetic mutations to arsenic trioxide
原文摘要:
Resistance to arsenic and/or all-trans retinoic acid (ATRA) is a challenging problem in the clinical management of acute promyelocytic leukemia (APL). Acquired genetic mutations in the PML moiety of the PML-RARA fusion gene are found in some patients with relapsed/refractory APL. Whether all of the identified point mutations play a role and have a similar function in the mechanisms of arsenic resistance remains unknown. Here we performed in vitro functional analyses and a retrospective analysis of APL patients to investigate the effect of PML-RARA mutations in mediating resistance to arsenic trioxide. Among the 5-point mutations in the PML part of PML-RARA identified in patients with relapsed APL, we found that A216V, S214L, and A216T mutations could attenuate the negative regulation of arsenic on PML-RARA, resulting in the retention of oncoproteins. In contrast, L217F and S220G mutations functioned weakly in this context. Furthermore, we demonstrated that either increasing the concentration of arsenic trioxide or combining it with ATRA could overcome the mutation-triggered arsenic resistance in vitro. In addition to presenting more evidence to reinforce the correlation of genetic mutations in PML-RARA with arsenic efficacy, we provide novel insight into the functional difference of acquired mutations of PML-RARA both in vitro and in the clinical setting. Our findings may help predict the prognosis and select more effective strategies during APL therapy.
DOI: http://dx.doi.org/10.1182/blood-2015-04-637678
作者:黄晓军

