Antiviral Res:中科院上海巴斯德所黄忠课题组发表柯萨奇病毒A16型
2016年2月21日,国际学术期刊《antiviral Research》在线发表了中科院上海巴斯德研究所黄忠课题组的科研成果“Coxsackievirus A16-like particles produced in Pichia pastoris elicit high-titer neutralizing antibodies and confer protection against lethal viral challenge in mice”(毕赤酵母生产的CA16病毒样颗粒诱导高效价中和抗体并保护小鼠免于致死剂量病毒攻击)。
手足口病是五岁以下儿童中常见的传染性疾病。柯萨奇病毒A16型(CA16)和肠道病毒71型(ev71)是引起手足口病的两个最主要的病原体,感染EV71或CA16都有可能引起严重的神经系统并发症甚至导致死亡。目前,EV71灭活疫苗已上市,而CA16疫苗研发相对缓慢,至今仍没有候选疫苗进入临床试验。
黄忠课题组长期致力于基于病毒样颗粒(VLP)的手足口病基因工程疫苗研发,在前期工作中已经利用毕赤酵母表达系统,完成了EV71 VLP疫苗的临床前有效性验证(Vaccine 2015, 33:2335-2341)。近期,课题组利用毕赤酵母系统开发CA16基因工程疫苗。博士研究生张超等在黄忠研究员和副研究员刘庆伟的指导下,在毕赤酵母中成功制备CA16 VLP,VLP能在小鼠中诱导出针对CA16的高效价中和性抗体,病毒攻击试验显示VLP疫苗免疫所产生的抗体能完全保护小鼠抵抗CA16病毒的感染。研究表明毕赤酵母来源的CA16 VLP是很好的CA16候选疫苗,具备成本低、操作简单及免疫原性好等优点,具有较好的产业化前景。
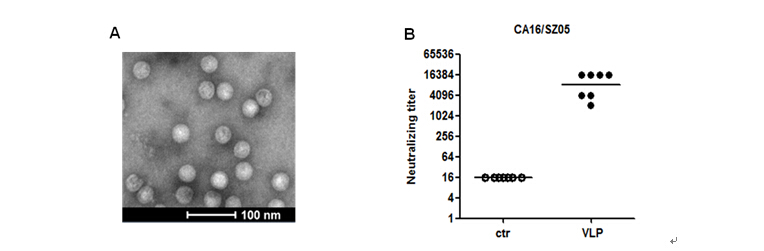
毕赤酵母来源的CA16 VLP在小鼠中诱导出高水平的中和抗体反应。(A)纯化的CA16 VLP的电镜照片。(B)CA16 VLP免疫小鼠血清对CA16/SZ05病毒株的中和效价。
原文链接:
Coxsackievirus A16-like particles produced in Pichia pastoriselicit high-titer neutralizing antibodies and confer protection against lethal viral challenge in mice
原文摘要:
Coxsackievirus A16 (CA16) is a major causative agent of hand, foot and mouse disease (HFMD) which has been affecting millions of young children annually in the Asia–Pacific region over the last seven years. However, no commercial CA16 vaccines are currently available. In the present study, we investigated the expression of virus-like particles (VLPs) of CA16 in Pichia pastoris yeast and their immunogenicity and protective efficacy in mice. We found that CA16-VLPs could be produced at relatively high levels in P. pastoris yeast transformed with a construct co-expressing the P1 and 3CD proteins of CA16. Mice immunized with the yeast-derived CA16-VLPs produced high-titer serum antibodies with potent neutralization effect specifically on CA16. More importantly, passive immunization with the yeast-derived VLPs fully protected neonatal mice against CA16 lethal challenge in both antisera transfer and maternal immunization experiments. Collectively, our results demonstrate that P. pastoris-derived CA16-VLPs represent a promising CA16 vaccine candidate with proven preclinical efficacy and desirable traits for manufacturing at industrial scale.
doi:10.1016/j.antiviral.2016.02.011
作者:黄忠

