J Control Release:中科大王均课题组通过纳米颗粒干预T细胞免疫检验
2016年01月29日,国际学术期刊《JouRNAl of Controlled Release》在线发表了中国科学技术大学生命科学学院王均教授课题组研究成果“Restoring anti-tumor functions of T cells via nanoparticle-mediated immune checkpoint modulation”。该研究发现通过纳米颗粒携载针对T细胞免疫检验点分子CTLA-4的小干扰RNA(siRNA)药物能够靶向肿瘤组织浸润T细胞,增强T细胞免疫功能和抗肿瘤免疫应答。
T细胞是介导抗肿瘤细胞免疫的核心效应细胞。细胞毒性T淋巴细胞相关抗原-4(Cytotoxic T Lymphocyte-associated Antigen-4,CTLA-4)是表达于活化T细胞表面的重要免疫检验点分子。CTLA-4与抗原提呈细胞(APC)上的配体B7结合后可以逆转T细胞抗原受体(TCR)活化导致的信号分子磷酸化,从而抑制T细胞活化增殖。T细胞免疫检验点信号通路常被肿瘤细胞巧妙利用,成为其诱导(肿瘤组织浸润)T细胞免疫耐受或无能、逃逸T细胞免疫监视的重要机制。随着纳米技术的发展,纳米载体系统已被广泛应用于改善和提高sirna等核酸类药物的药代动力学,进而提高肿瘤治疗效果和降低药物系统副作用。利用纳米载体递送siRNA药物,干预阻断T细胞免疫检验点有望释放(肿瘤组织浸润)T细胞抗肿瘤免疫反应潜能,增强T细胞免疫应答能力,为肿瘤治疗提供新的策略。
王均课题组使用一种基于聚乙二醇-聚乳酸的阳离子脂质辅助的纳米颗粒输送抑制T细胞表面免疫抑制性受体分子CTLA-4的siRNA(NPsiCTLA-4)用于增强由纳米药物载体辅助的T细胞杀伤恶性黑色素瘤细胞功能,促进肿瘤免疫治疗效果。在该研究中,NPsiCTLA-4能够携载针对CTLA-4的siRNA药物(siCTLA-4)有效进入体外培养的T细胞并下调T细胞表面CTLA-4的表达。在恶性黑色素瘤小鼠模型中,通过系统给药输送NPsiCTLA-4可有效增加肿瘤部位浸润的效应性CD8+ T细胞和CD4+ T细胞的数目,并促进其活化和增殖,同时降低具有免疫抑制作用的调节性T细胞(Treg)比例,使得肿瘤部位处于一个抗肿瘤的细胞免疫微环境,进而达到抑制肿瘤生长、延长小鼠存活期的目的。
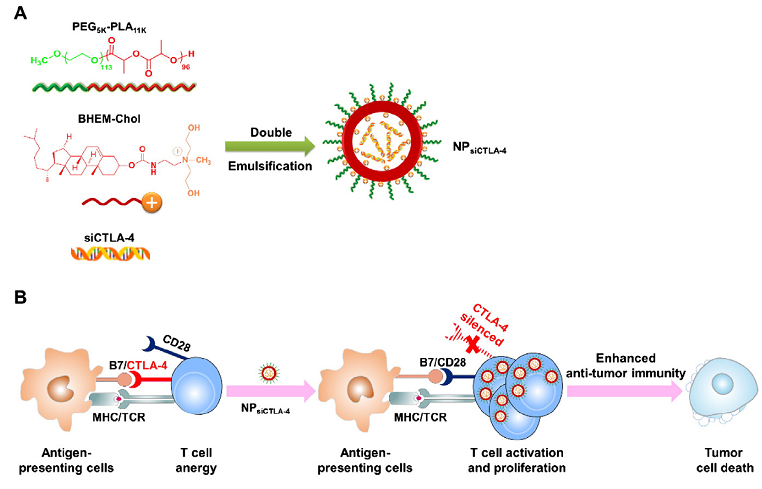
图1、携载了siCTLA-4的纳米药物载体(NPsiCTLA-4)(A)通过系统给药后,可靶向体内t细胞(包括肿瘤浸润T细胞),有效降低T细胞表面CTLA-4的表达、解除T细胞免疫功能抑制状态(无能或耗竭)以及促进活化T细胞的持续激活与增殖,增强T细胞抗肿瘤免疫杀伤功能(B)。
原文链接:
Restoring anti-tumor functions of T cells via nanoparticle-mediated immune checkpoint modulation
原文摘要:
The core purpose of cancer immunotherapy is the sustained activation and expansion of the tumor specific T cells, especially tumor-infiltrating cytotoxic T lymphocytes (CTLs). Currently, one of the main foci of immunotherapy involving nano-sized carriers is on cancer vaccines and the role of professional antigen presenting cells, such as dendritic cells (DCs) and other phagocytic immune cells. Besides the idea that cancer vaccines promote T cell immune responses, targeting immune inhibitory pathways with nanoparticle delivered regulatory agents such as small interfering RNA (siRNA) to the difficultly-transfected tumor-infiltrating T cells may provide more information on the utility of nanoparticle-mediated cancer immunotherapy. In this study, we constructed nanoparticles to deliver cytotoxic T lymphocyte-associated molecule-4 (CTLA-4)-siRNA (NPsiCTLA-4) and showed the ability of this siRNA delivery system to enter T cells both in vitro and in vivo. Furthermore, T cell activation and proliferation were enhanced after NPsiCTLA-4 treatment in vitro. The ability of direct regulation of T cells of this CTLA-4 delivery system was assessed in a mouse model bearing B16 melanoma. Our results demonstrated that this nanoparticle delivery system was able to deliver CTLA-4-siRNA into both CD4+ and CD8+ T cell subsets at tumor sites and significantly increased the percentage of anti-tumor CD8+ T cells, while it decreased the ratio of inhibitory T regulatory cells (Tregs) among tumor infiltrating lymphocytes (TILs), resulting in augmented activation and anti-tumor immune responses of the tumor-infiltrating T cells. These data support the use of potent nanoparticle-based cancer immunotherapy for melanoma.
doi:10.1016/j.jconrel.2016.01.044
作者:王均

