Vaccine:中科院上海巴斯德所黄忠研究组发表肠道病毒71和诺如病毒
9月28日,国际学术期刊Vaccine 在线发表了中国科学院上海巴斯德研究所疫苗中心黄忠研究组的研究论文A bivalent virus-like particle based vaccine induces a balanced antibody response against both enterovirus 71 and norovirus in mice(《基于病毒样颗粒的肠道病毒71型和诺如病毒联合疫苗在小鼠上引起均衡的抗体反应》),王晓丽为论文第一作者,黄忠研究员为论文通讯作者,该研究得到了上海巴斯德所疫苗中心主任金侠及国家蛋白质中心电镜室的大力支持。
肠道病毒71型(EV71)和诺如病毒(NoV)是两种经消化道感染的RNA病毒,在儿童中的感染率极高。据估计,仅在发展中国家,诺如病毒感染每年导致约20万儿童死亡,其基因型II.4(GII.4)是诺如病毒最主要的致病原。EV71是引发儿童手足口病的重要病原体,重症感染会出现严重的神经及呼吸系统并发症,甚至死亡。至今尚无抗GII.4或抗EV71的商业化疫苗。因此,开发针对GII.4和EV71的疫苗,尤其是联合疫苗,对于保障儿童生命健康具有重要的现实意义。
上海巴斯德所疫苗学和抗病毒策略研究组博士生王晓黎等在研究员黄忠的指导下,利用已建立的病毒样颗粒(VLP)表达平台(PLoS ONE 2013;Vaccine 2012;Vaccine 2014)制备EV71-VLP和GII.4-VLP,构建了包含两种VLP的二联疫苗(Bi-VLP)并进行系统的免疫效应研究。结果显示,联合疫苗Bi-VLP可以诱导针对EV71和GII.4的抗体反应,且诱导的抗体水平和持久性与单价VLP疫苗相当,表明联合疫苗中的两种VLP组分之间不存在免疫干扰。更重要的是,Bi-VLP组小鼠血清不仅可以有效地中和EV71病毒,还能阻断GII.4-VLP与其细胞表面结合分子的相互作用,提示该血清对两种病毒都有预防作用。此项研究明确了基于VLP的抗EV71/GII.4二联疫苗的可行性和有效性,为进一步的临床前和临床研究奠定了基础。
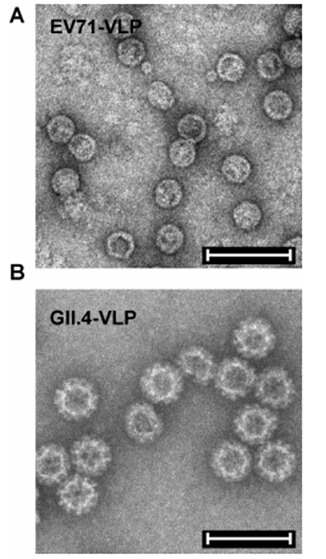
VLP电镜图(A)ev71-VLP, (B) GII.4-VLP Bar=100nm
原文链接:
A bivalent virus-like particle based vaccine induces a balanced antibody response against both enterovirus 71 and norovirus in mice
原文摘要:
Noroviruses are the main cause of severe viral gastroenteritis, which results in estimated 200,000 deaths each year, primarily in children in the developing world. Genogroup II.4 (GII.4) strains are responsible for the majority of norovirus outbreaks. Enterovirus 71 (EV71), the leading causative agent of hand, foot and mouth disease, has recently been prevalent in Asia-Pacific regions, resulting in significant morbidity and mortality in young children. However, no vaccine is commercially available for either norovirus GII.4 or EV71. Recombinant virus-like particles (VLPs) derived from either GII.4 or EV71 have been shown to be promising monovalent vaccine candidates. In this study, we investigate the possibility to formulate a VLP-based bivalent vaccine for both norovirus GII.4 and EV71. The GII.4- and EV71-VLPs were produced in a baculovirus-insect cell expression system. A bivalent combination vaccine comprised of GII.4 and EV71 VLPs was formulated and compared with monovalent GII.4- and EV71-VLPs for their immunogenicity in mice. We found that the bivalent vaccine elicited durable antibody responses toward both GII.4 and EV71, and the antibody titers were comparable to that induced by the monovalent vaccines, indicating there is no immunological interference between the two antigens in the combination vaccine. More significantly, the bivalent vaccine-immunized mouse sera could efficiently neutralize EV71 infection and block GII.4-VLP binding to mucin. Together, our results demonstrate that the experimental combination vaccine comprised of GII.4 and EV71-VLPs is able to induce a balanced protective antibody response, and therefore strongly support further preclinical and clinical development of such a bivalent VLP vaccine targeting both norovirus GII.4 and EV71.
doi:10.1016/j.vaccine.2015.09.043
作者:黄忠

