RNA Biology:中科院生化与细胞所科研人员揭示甲基转移酶家族成
6月24日,国际学术期刊RNA biology在线发表了中国科学院上海生命科学研究院生物化学与细胞生物学研究所王恩多研究组题为“Identification of determinants for tRNA substrate recognition by Escherichia coli C/U34 2'-O-methyltransferase”的研究论文。
tRNA是细胞内主要的RNA之一,它的经典功能是参与蛋白质合成。tRNA上的核苷酸存在着广泛的转录后修饰,尤其是位于tRNA的反密码子环上的核苷酸残基,这些修饰对于tRNA在细胞内发挥功能起着重要作用,缺失某些修饰将引起细胞的严重缺陷。原核生物中,TrmL负责tRNALeuCAA和tRNALeuUAA两种等受体的反密码子环上第34位核苷酸的甲基化修饰。TrmL是SPOUT甲基转移酶家族最小成员之一,其对tRNA识别的关键元件并不清楚。
在王恩多研究员和刘如娟副研究员的指导下,博士研究生周觅等人通过定点突变、结合酶学动力学等生物化学手段鉴定出了tRNA上参与TrmL识别的关键元件。研究发现TrmL不依赖于tRNA全长的倒L型三级结构,仅需要识别带有特定修饰的反密码子茎环。TrmL伸出许多手臂同时识别tRNA上不同元件,用来催化tRNA34位核苷酸在核糖上2'-羟基的甲基化。具体识别元件为: (1) tRNA反密码子环上特定核苷酸序列; (2) 反密码子茎环外加两对碱基对是TrmL识别的最小底物;(3) 被催化的34位摆动位点为嘧啶核苷酸;(4)第37位A上的异戊烯基化修饰。 该工作得到了国家基础研究基金、国家自然科学基金、中科院、上海市科委等的资助。
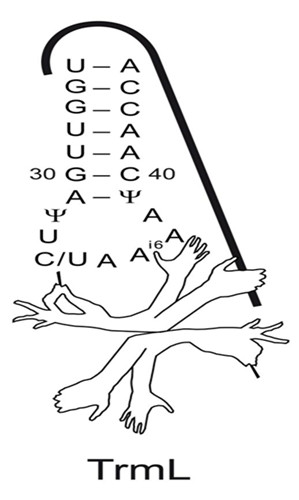
TrmL识别底物tRNA的关键元件示意图
原文链接:
Identification of determinants for tRNA substrate recognition by Escherichia coli C/U34 2'-O-methyltransferase.
原文摘要:
Post-transcriptional modifications bring chemical diversity to tRNAs, especially at positions 34 and 37 of the anticodon stem-loop (ASL). TrmL is the prokaryotic methyltransferase that catalyzes the transfer of the methyl group from S-adenosyl-L-methionine to the wobble base of tRNALeuCAA and tRNALeuUAA isoacceptors. This Cm34/Um34 modification affects codon-anticodon interactions and is essential for translational fidelity. TrmL-catalyzed 2'-O-methylation requires its homodimerization; however, understanding of the tRNA recognition mechanism by TrmL remains elusive. In the current study, by measuring tRNA methylation by TrmL and performing kinetic analysis of tRNA mutants, we found that TrmL exhibits a fine-tuned tRNA substrate recognition mechanism. Anticodon stem-loop minihelices with an extension of two base pairs are the minimal substrate for EcTrmL methylation. A35 is a key residue for TrmL recognition, while A36-A37-A38 are important either via direct interaction with TrmL or due to the necessity for prior isopentenylation (i6) at A37. In addition, TrmL only methylates pyrimidines but not purine residues at the wobble position, and the 2'-O-methylation relies on prior N6-isopentenyladenosine modification at position 37.
DOI:10.1080/15476286.2015.1050576
作者:王恩多

