The Plant Cell:上海生科院揭示玉米种子储存蛋白表达调控的分子
4月21日,The Plant Cell 杂志在线发表了中国科学院上海生命科学研究院植物生理生态研究所巫永睿研究组题为Transcriptional Regulation of Zein Gene Expression in Maize through the Additive and Synergistic Action of opaque2, Prolamine-Box Binding Factor, and O2 Heterodimerizing Proteins 的研究论文。该研究论文报道了玉米籽粒中表达量最高的种子储存蛋白——醇溶蛋白表达调控的最新研究进展。
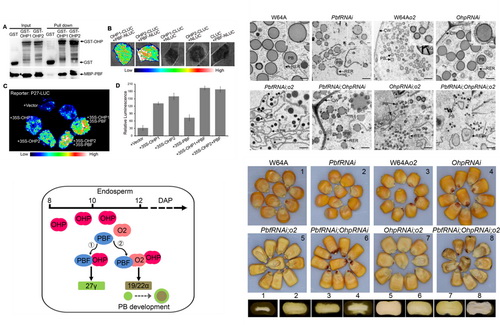
玉米作为全世界第一大作物,其种子含有约10%的蛋白质,其中60%以上为醇溶蛋白,通称为zein。虽然Zein的高水平表达对籽粒胚乳硬度起关键性作用,但是它们几乎不含必须氨基酸——赖氨酸和色氨酸。因此,普通玉米的营养品质很差。Zein作为一类只在玉米胚乳灌浆期特异高表达的种子储存蛋白,其家族成员根据蛋白序列同源性,分为α,β,γ和δ四大类,上世纪80年代,它们被陆续克隆鉴定。opaque2 (O2)是第一个被鉴定的调控醇溶蛋白表达的转录因子(Schmidt et al., 1987),属于bZIP转录因子家族,通过结合到顺式作用元件O2-box (TCCACGT),启动22-kD α-zein等的表达(Schmidt et al., 1992)。O2 Heterodimerizing Protein 1 和2 (OHP1和OHP2) 是通过筛cDNA库鉴定到的另外两个bZIP转录因子,它们能和O2形成异源二聚体,但功能一直不清楚;Prolamin-box Binding Factor (PBF)是第二个被鉴定的调控醇溶蛋白表达的转录因子,属于Dof转录因子家族(Vicente-Carbajosa et al., 1997),该转录因子通过结合到顺式作用元件P-box (TGTAAAG),调控27-kD γ-zein表达 (Wu and Messing, 2012)。
该研究利用生化和遗传手段发现OHP1和OHP2通过结合到顺式作用元件O2-like box (TTTACGT),与PBF相互作用共同调控27-kD γ-zein表达。通过构建o2,PbfRNAi 和 OhpRNAi 不同组合的双突变和三突变,揭示这三个转录因子调控了90%的醇溶蛋白表达。该研究结合α-zein和γ-zein在胚乳蛋白体形成过程中的时空表达模式,进一步提出了这三个转录因子调控蛋白体形成的分子模型。这一研究进一步推进了人们对玉米籽粒醇溶蛋白合成及其它作物种子储存蛋白表达调控分子机理的认识。
该研究工作在国家自然科学基金(31371630, 91335109和31422040)和中组部青年千人计划的资助下,由助理研究员张志勇和博士后杨俊在研究员巫永睿指导下共同完成。
上海生科院揭示玉米种子储存蛋白表达调控的分子机理
原文链接:
Transcriptional Regulation of Zein Gene expression in Maize through the Additive and Synergistic Action of opaque2, Prolamine-Box Binding Factor, and O2 Heterodimerizing Proteins
原文摘要:
Maize (Zea mays) zeins are some of the most abundant cereal seed storage proteins (SSPs). Their abundance influences kernel hardness but compromises its nutritional quality. Transcription factors regulating the expression of zein and other SSP genes in cereals are endosperm-specific and homologs of maize opaque2 (O2) and prolamine-box binding factor (PBF). This study demonstrates that the ubiquitously expressed transcription factors, O2 heterodimerizing proteins (OHPs), specifically regulate 27-kD γ-zein gene expression (through binding to an O2-like box in its promoter) and interact with PBF. The zein content of double mutants OhpRNAi;o2 and PbfRNAi;o2 and the triple mutantPbfRNAi;OhpRNAi;o2 is reduced by 83, 89, and 90%, respectively, compared with the wild type. The triple mutant developed the smallest zein protein bodies, which were merely one-tenth the wild type’s size. Total protein levels in these mutants were maintained in a relatively constant range through proteome rebalancing. These data show that OHPs, O2, and PBF are master regulators of zein storage protein synthesis, acting in an additive and synergistic mode. The differential expression patterns of OHP and O2 genes may cause the slight differences in the timing of 27-kD γ-zein and 22-kD α-zein accumulation during protein body formation.
作者:生物帮

