武汉病毒所首次实现动物活体内RNA-蛋白质相互作用的荧光成像
中国科学院武汉病毒研究所崔宗强研究员和中科院生物物理研究所张先恩研究员联合研究团队在蛋白-蛋白及RNA-蛋白质相互作用的活体内分子成像方面取得重要进展,创建了远红光波段的荧光片段互补系统(Far-red fluorescence complementation systems),首次实现了动物活体内RNA-蛋白质相互作用的荧光成像,并揭示了HIV-1 mRNA与人PTB蛋白相互作用机制。相关研究结果近日已在线发表于核心期刊Nucleic Acids Research。
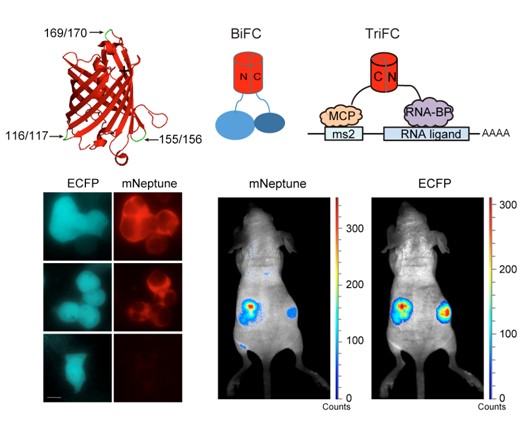
蛋白-蛋白及RNA-蛋白质相互作用的活体成像一直是挑战性的研究课题。该研究中,研究团队开发了激发波长在600 nm以上的红色荧光蛋白mNeptune荧光片段互补技术,建立了位于活体成像“光学窗口”的双分子和三分子荧光互补系统。其中双分子荧光互补系统可以用于活细胞及活体内蛋白-蛋白相互作用成像,三分子荧光互补系统可以用于活细胞及活体内RNA-蛋白质之间的相互作用成像。利用该新型荧光互补系统,针对可能参与艾滋病毒“潜伏-激活”过程的人PTB蛋白与病毒mRNA之间的相互作用研究,发现HIV-1病毒mRNA的3’LTR区域与PTB蛋白有特异性的相互作用,并在活细胞及活体内对其相互作用进行了影像分析。
该研究首次建立了活体内RNA-蛋白质相互作用荧光成像技术,该技术也将有助于开发活细胞及活体内药物评价体系和药物筛选平台。艾滋病毒mRNA与PTB蛋白相互作用解析也将为理解和调控病毒“潜伏-激活”过程提供新思路。
原文摘要:
Yu Han, Shifeng Wang, Zhiping Zhang, Xiaohe Ma, Wei Li,Xiaowei Zhang, Jiaoyu Deng, Hongping Wei, Zhaoyang Li,Xian-En Zhang and Zongqiang Cui
Imaging of protein–protein and RNA–protein interactions in vivo, especially in live animals, is still challenging. Here we developed far-red mNeptune-based bimolecular fluorescence complementation (BiFC) and trimolecular fluorescence complementation (TriFC) systems with excitation and emission above 600 nm in the ‘tissue optical window’ for imaging of protein–protein and RNA–protein interactions in live cells and mice. The far-red mNeptune BiFC was first built by selecting appropriate split mNeptune fragments, and then the mNeptune-TriFC system was built based on the mNeptune-BiFC system. The newly constructed mNeptune BiFC and TriFC systems were verified as useful tools for imaging protein–protein and mRNA–protein interactions, respectively, in live cells and mice. We then used the new mNeptune-TriFC system to investigate the interactions between human polypyrimidine-tract-binding protein (PTB) and HIV-1 mRNA elements as PTB may participate in HIV mRNA processing in HIV activation from latency. An interaction between PTB and the 3′long terminal repeat region of HIV-1 mRNAs was found and imaged in live cells and mice, implying a role for PTB in regulating HIV-1 mRNA processing. The study provides new tools for in vivo imaging of RNA–protein and protein–protein interactions, and adds new insight into the mechanism of HIV-1 mRNA processing.
作者:武汉病毒所

