基因组所利用计算机分析TALE蛋白特异结合DNA的机制
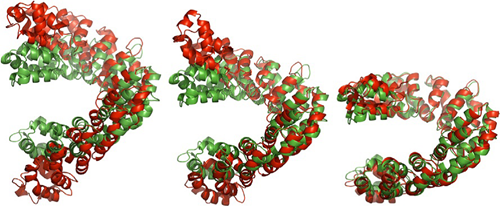
三个具有代表性的分子动力学模拟轨迹。其中红色为模拟的结构,绿色为TALE蛋白结合DNA的参照结构
TALE蛋白是一个序列可编程的转录因子,由34个氨基酸的重复序列组成。每个重复序列形成两个螺旋,这两个螺旋通过一个短的环(Loop)连接。Loop内含有重复数可变的双氨基酸(Repeat-variable di-residues,RVDs),可以特异性识别DNA碱基。尽管晶体结构描述了TALE蛋白在开放状态和结合DNA状态之间的转变,但这两种构象如何在较低的能量消耗下实现转换以及RVDs识别DNA碱基的机制都不清楚。
为此,雷红星研究员及其研究团队围绕着以上两个方面对TALE-DNA相互作用进行了计算分析,以便更好地理解相互作用过程中能量的变化。在对结合DNA和自由的构象分别进行分子动力学模拟后,研究人员均发现TALE蛋白具有很高的弹性,而且两者之间有很低的自由能势垒,只需要很少的能量TALE蛋白就能结合DNA。
与此同时,为了分析TALE蛋白识别DNA的特异性,研究人员利用PBSA(Poisson–Boltzmann surface area)以及其他方法去计算RVDs和DNA碱基多种组合的自由能。PBSA计算结果表明,正确的RVDs-碱基配对与错误的配对相比具有较低的自由能。
在这些分析的基础上,该理论分析为理解TALE蛋白结合DNA的动态过程以及伴随的能量变化提供了新的视野。
原文摘要:
Chapter Nine – Conformational Elasticity can Facilitate TALE–DNA Recognition
Hongxing Lei,Jiya Sun,Enoch P. Baldwin,David J. Sega,Yong Duan
Sequence-programmable transcription activator-like effector (TALE) proteins have emerged as a highly efficient tool for genome engineering. Recent crystal structures depict a transition between an open unbound solenoid and more compact DNA-bound solenoid formed by the 34 amino acid repeats. How TALEs switch conformation between these two forms without substantial energetic compensation, and how the repeat-variable di-residues (RVDs) discriminate between the cognate base and other bases still remain unclear. Computational analysis on these two aspects of TALE–DNA interaction mechanism has been conducted in order to achieve a better understanding of the energetics. High elasticity was observed in the molecular dynamics simulations of DNA-free TALE structure that started from the bound conformation where it sampled a wide range of conformations including the experimentally determined apo and bound conformations. This elastic feature was also observed in the simulations starting from the apo form which suggests low free energy barrier between the two conformations and small compensation required upon binding. To analyze binding specificity, we performed free energy calculations of various combinations of RVDs and bases using Poisson–Boltzmann surface area (PBSA) and other approaches. The PBSA calculations indicated that the native RVD–base structures had lower binding free energy than mismatched structures for most of the RVDs examined. Our theoretical analyses provided new insight on the dynamics and energetics of TALE–DNA binding mechanism.
标签: DNA TALE蛋白 分子动力学模拟
作者:基因组所

