Nature:德美科学家合作新开发一种可获得微小蛋白晶体结构技术
德国马克斯 - 普朗克医学研究所和美国SLAC国家加速器实验室的研究人员新研发一种可获得微小蛋白的方法。相关文章发表于2013年11月24日的《Nature》杂志上。
Nature:德美科学家合作新开发一种可获得微小蛋白晶体结构技术
X-射线晶体学研究人员一般会花很多时间优化结晶条件,来获得产生高质量数据集所需的大的、非常有序的晶体。最近的研究表明,来自X-射线自由电子激光器的极短的、强烈的X-射线脉冲,可被用来在晶体的辐射损伤发生之前获得关于纳米到微米大小的蛋白晶体的数据。
研究人员希望,这种方法(被称为“序列飞秒晶体学”方法)将能产生不会形成宏观的、非常有序的晶体的蛋白和蛋白复合物的结构。“序列飞秒晶体学”方法的一大局限性是,在没有事先知道一种蛋白的相关已知结构的情况下,此前一直没有可能确定其结构。
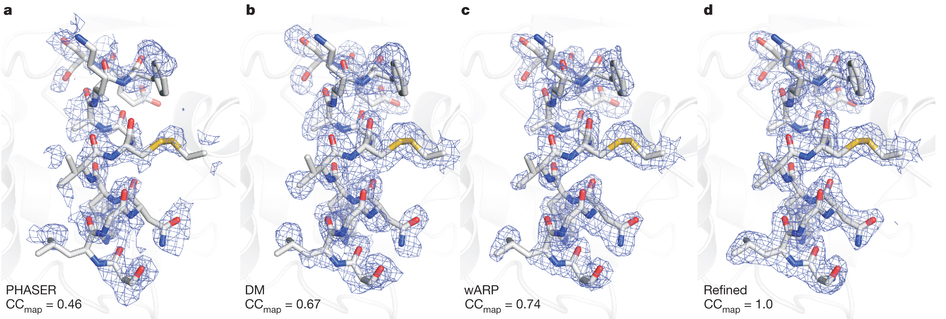
在这篇论文中,作者介绍了“序列飞秒晶体学”方法何以能够与X-射线自由电子激光相结合,被用来通过实验解决“相位问题”,在没有事先知道一种蛋白是什么样的情况下生成它的高分辨率结构。
原文摘要:
De novo protein crystal structure determination from X-ray free-electron laser data
Thomas R. M. Barends, Lutz Foucar, Sabine Botha, R. Bruce Doak, Robert L. Shoeman,Karol Nass, Jason E. Koglin, Garth J. Williams, Sébastien Boutet, Marc Messerschmidt &Ilme Schlichting
The determination of protein crystal structures is hampered by the need for macroscopic crystals. X-ray free-electron lasers (FELs) provide extremely intense pulses of femtosecond duration, which allow data collection from nanometre- to micrometre-sized crystals in a ‘diffraction-before-destruction’ approach. So far, all protein structure determinations carried out using FELs have been based on previous knowledge of related, known structures. Here we show that X-ray FEL data can be used for de novo protein structure determination, that is, without previous knowledge about the structure. Using the emerging technique of serial femtosecond crystallography, we performed single-wavelength anomalous scattering measurements on microcrystals of the well-established model system lysozyme, in complex with a lanthanide compound. Using Monte-Carlo integration, we obtained high-quality diffraction intensities from which experimental phases could be determined, resulting in an experimental electron density map good enough for automated building of the protein structure. This demonstrates the feasibility of determining novel protein structures using FELs. We anticipate that serial femtosecond crystallography will become an important tool for the structure determination of proteins that are difficult to crystallize, such as membrane proteins.

