北京大学药学院屠鹏飞团队揭示中药作用新靶点

在国家自然科学基金项目等资助下,北京大学药学院天然药物与仿生药物国家重点实验室教授屠鹏飞团队研究揭示了传统中药苏木的抗神经炎症活性成分苏木酮A的直接作用靶点蛋白为肌苷-5-单磷酸脱氢酶2(IMPDH2),相关研究成果在《美国科学院院刊》上在线发表。
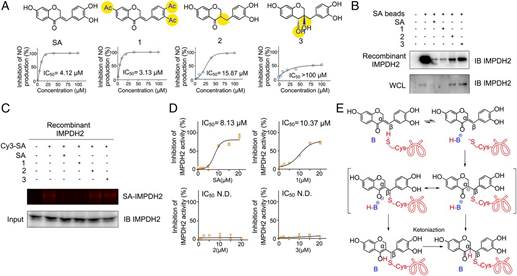
屠鹏飞团队将当前化学生物学领域的分子探针概念引入到中药活性成分的靶点鉴定中,结果发现肌苷-5-单磷酸脱氢酶2(IMPDH2)是其发挥抗神经炎症作用的一个关键靶点。进一步研究又发现,苏木酮A可以通过对靶点蛋白IMPDH2的共价修饰,进而诱导其变构失活,抑制靶点蛋白下游的多条炎症相关信号通路的激活,最终实现抗神经炎症作用。这一研究成果不仅揭示了中药活性成分苏木酮A的抗炎作用机理,同时也在IMPDH2蛋白上发现了一个全新的药物作用位点,对于今后以IMPDH2蛋白为靶点的抗炎和免疫抑制药物的设计和研发具有指导意义。(来源:中国科学报 陈欢欢)
Highly selective inhibition of IMPDH2 provides the basis of antineuroinflammation therapy
Abstract Inosine monophosphate dehydrogenase (IMPDH) of human is an attractive target for immunosuppressive agents. Currently, small-molecule inhibitors do not show good selectivity for different IMPDH isoforms (IMPDH1 and IMPDH2), resulting in some adverse effects, which limit their use. Herein, we used a small-molecule probe specifically targeting IMPDH2 and identified Cysteine residue 140 (Cys140) as a selective druggable site. On covalently binding to Cys140, the probe exerts an allosteric regulation to block the catalytic pocket of IMPDH2 and further induces IMPDH2 inactivation, leading to an effective suppression of neuroinflammatory responses. However, the probe does not covalently bind to IMPDH1. Taken together, our study shows Cys140 as a druggable site for selectively inhibiting IMPDH2, which provides great potential for development of therapy agents for autoimmune and neuroinflammatory diseases with less unfavorable tolerability profile.
原文链接:http://www.pnas.org/content/114/29/E5986.full.pdf

