高通量方法可以在黑暗中测量植物线粒体呼吸的速率
植物和动物一样,也在日夜不停地呼吸。尽管植物没有明显的呼吸器官,但根茎叶的每一个细胞都在呼吸。这个过程消耗大量氧气,并释放出二氧化碳。然而,植物学家总是缺乏一种有效方法,来测定不同物种之间的这个能量生产过程如何变化。
近日,澳大利亚国立大学的研究人员首次开发出一种高通量方法,可以在黑暗中测量植物线粒体呼吸的速率。这种方法发表在《Plant Methods》杂志上,可以提供数据,帮助人们追踪气候变化,并改善作物开发。
“每年,植物通过光合作用吸收1200亿吨的碳,但通过呼吸作用释放一半的量,远远超过燃烧化石燃料所释放的60-80亿吨。如果我们想预测未来几十年大气中的碳积累,我们就需要解释植物的呼吸过程,”这篇文章的通讯作者Owen Atkin解释说。
黑暗状态下的线粒体呼吸(Rdark)是一个关键的植物生理过程,因此,准确、高效地测定Rdark的速率变化对农艺和生态学研究很关键。然而,目前测定植物组织中Rdark的方法通常都是低通量的,无法满足当前研究人员的需求。因此,Atkin领导的研究小组开发出一种自动荧光系统,可检测多个氧气消耗率。
研究人员从三种不同的植物中采集了根、茎和叶组织样品,并将它们放在有专用盖的密封管中,其中含有对氧敏感的荧光染料。他们利用机器人系统扫描了盖子上的荧光信号,并记录氧浓度随着时间的推移而下降的水平。
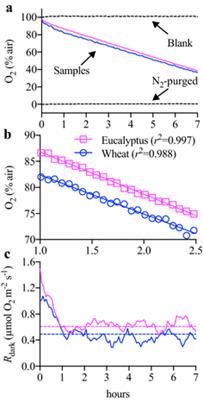
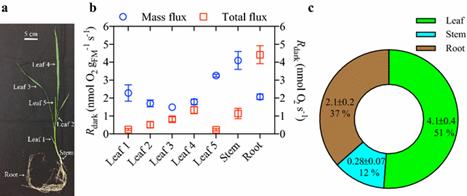
这种高通量的荧光系统能够在多个小时内稳定测量多个组织中的Rdark。与现有的方法相比,新方法快了10-26倍。这种技术也有着多方面的应用。它可以快速筛选138种小麦基因型的Rdark,也可以通过地上和地下器官的同时测定,定量整个植物的Rdark,而这是目前难以实现的。
另一名作者、来自西澳大利亚大学的Brendan O’Leary表示:“我们发现了物种内和物种之间的大量呼吸变化,这让我们对它的筛选潜力感到兴奋。育种人员现在可以对种子大小、种子数量和生长速率进行遗传选择,但快速测定不同作物的呼吸效率,这又增加了一个新层面。”
研究人员表示,他们正利用此系统来分析可能与呼吸变化相关的代谢物、蛋白质以及遗传学。他们希望这可以为小麦育种开辟新方法,以提高整体的粮食产量。(来源:生物通 薄荷)
The combination of gas-phase fluorophore technology and automation to enable high-throughput analysis of plant respiration
Abstract
Background Mitochondrial respiration in the dark (R dark) is a critical plant physiological process, and hence a reliable, efficient and high-throughput method of measuring variation in rates of R dark is essential for agronomic and ecological studies. However, currently methods used to measure R dark in plant tissues are typically low throughput. We assessed a high-throughput automated fluorophore system of detecting multiple O2consumption rates. The fluorophore technique was compared with O2-electrodes, infrared gas analysers (IRGA), and membrane inlet mass spectrometry, to determine accuracy and speed of detecting respiratory fluxes. Results The high-throughput fluorophore system provided stable measurements of R dark in detached leaf and root tissues over many hours. High-throughput potential was evident in that the fluorophore system was 10 to 26-fold faster per sample measurement than other conventional methods. The versatility of the technique was evident in its enabling: (1) rapid screening of R dark in 138 genotypes of wheat; and, (2) quantification of rarely-assessed whole-plant R dark through dissection and simultaneous measurements of above- and below-ground organs.
Discussion Variation in absolute R dark was observed between techniques, likely due to variation in sample conditions (i.e. liquid vs. gas-phase, open vs. closed systems), indicating that comparisons between studies using different measuring apparatus may not be feasible. However, the high-throughput protocol we present provided similar values of R dark to the most commonly used IRGA instrument currently employed by plant scientists. Together with the greater than tenfold increase in sample processing speed, we conclude that the high-throughput protocol enables reliable, stable and reproducible measurements of R dark on multiple samples simultaneously, irrespective of plant or tissue type.
原文链接:https://plantmethods.biomedcentral.com/articles/10.1186/s13007-017-0169-3

