The Plant Journal:中科院植生所郭房庆研究组揭示高温胁迫下植物热
2016年12月10日,国际植物学权威期刊《The plant Journal》在线发表了中国科学院上海生命科学研究院植物生理生态研究所郭房庆研究组题为“Identification of Core Subunits of Photosystem II as Action Sites of HSP21 That Is Activated by the GUN5-Mediated Retrograde Pathway in Arabidopsis”的最新研究论文。研究结果揭示HSP21作为分子伴侣蛋白通过与光系统II复合体(photosystem II, PSII)核心亚基蛋白(如D1和D2等)的直接结合,维持高温胁迫下PSII复合体及类囊体膜的稳定性,进而提高植物高温胁迫下光合效率及存活率。博士生陈思婷为论文第一作者,郭房庆研究员为论文通讯作者。
光合作用在植物细胞叶绿体中进行。高温抑制光合作用主要的原因是造成“栖息”在叶绿体类囊体膜上光合复合体蛋白的迅速降解,叶片光合机能丧失,进而导致作物严重减产或最终死亡。因此,解析植物在高温胁迫下如何维持其光合复合体稳定性的分子机理,对于提高植物高温抗性、增加产量具有重要意义。植物应对高温胁迫的反应是启动体内大量热激蛋白(heat shock protein,HSP)的合成,其中包括核基因编码且定位于叶绿体的小热激蛋白HSP21。
郭房庆研究组通过多种活体和离体蛋白互作验证手段和体系的运用,明确了PSII核心亚基为HSP21的保护靶点蛋白。令人意想不到的是,蛋白互作验证结果表明HSP21与光系统I (PSI)核心亚基PsaA和PsaB未呈现明显的互作特征,表明HSP21对于叶绿体光合复合体的保护作用具有选择性。前人研究结果表明,相较于其它复合体,PSII光合复合体对于高温胁迫最为敏感。因此,HSP21优先选择性保护PSII从植物适应高温胁迫进化机制上是可以理解的。为了进一步定量分析HSP21与保护靶蛋白的结合特性,研究人员利用微量热泳动技术(Microscale thermophoresis)深入解析了HSP21与D1和D2的结合动力学特征以及结合常数。同时,通过电镜免疫组化技术,证实HSP21同时定位于叶绿体类核区和类囊体膜上,为阐释HSP21的保护功能提供了坚实的细胞学基础。
研究组前期研究首次证实高等植物细胞存在热激反应的叶绿体逆向(retrograde)调控信号途径(Yu et al., 2012; Sun and Guo, 2016)。该项研究进一步证实,HSP21作为典型的热激响应基因,其高温诱导表达受叶绿体逆向调控途径关键组分GUN5的调控。突变体遗传证据显示,组成型表达HSP21可以显著提高高温胁迫敏感突变体gun5的存活率。上述研究进展为细胞核-质体信号互做参与植物高温逆境胁迫适应机制提供了新的证据,进一步丰富和完善了植物细胞热激反应的叶绿体逆向调控机制模型。
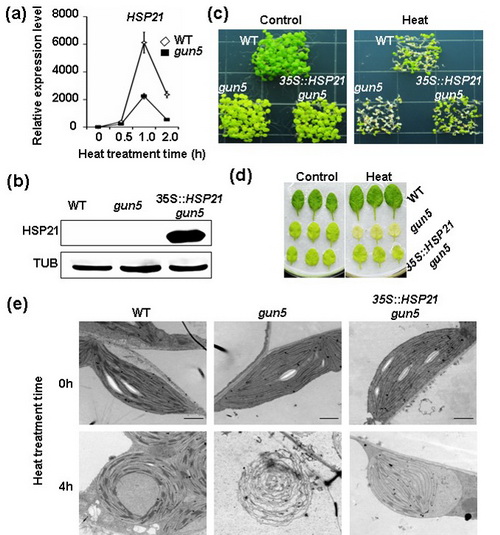
过表达HSP21显著增强gun5突变体高温抗性示意图
原文链接:
Identification of Core Subunits of Photosystem II as Action Sites of HSP21 That Is Activated by the GUN5-Mediated Retrograde Pathway in ArABIdopsis
原文摘要:
Photosystem II (PSII) is the most thermolabile photosynthetic complex. Physiological evidence suggests that the small chloroplast heat-shock protein 21 (HSP 21) is involved in plant thermotolerance but the molecular mechanism of its action remains largely unknown. Here, we have provided genetic and biochemical evidence that HSP 21 is activated by the GUN5-dependent retrograde signaling pathway and stabilizes PSII by directly binding to its core subunits such as D1 and D2 proteins under heat stress. We further demonstrate that the constitutive expression of HSP21sufficiently rescues the thermo-sensitive stability of PSII and survival defects of thegun5 mutant with dramatically improving granal stacking under heat stress, indicating that HSP21 is a master chaperone protein in maintaining the integrity of thylakoid membrane system under heat stress. In line with our interpretation based on several lines of in vitro and in vivo protein-interaction evidence that HSP21 interacts with core subunits of PSII, the kinetics of HSP21 binding to the D1 and D2 proteins was determined by performing analysis of microscale thermophoresis. Considering a major role of HSP21 in protecting the core subunits of PSII from thermal damages, its heat-responsive activation via the heat shock transcription factor HsfA2 is critical for the survival of plants under heat stress. Our findings reveal an auto-adaptation loop pathway that Plant Cells optimize particular needs of chloroplasts in stabilizing photosynthetic complexes by relaying the GUN5-dependent plastid signal(s) to activate the heat-responsive expression of HSP21 in the nucleus during adaptation to heat stress in plants.
作者:郭房庆

