PLOS Pathogens:中科院武汉病毒所陈新文研究组揭示杆状病毒特异性
2016年11月1日,国际微生物学知名期刊《PLOS Pathogens》在线发表了中国科学院武汉病毒研究所陈新文研究组题为“Autographa californica Multiple Nucleopolyhedrovirus Ac34 Protein Retains Cellular Actin-Related Protein 2/3 Complex in the Nucleus by Subversion of CRM1-Dependent Nuclear Export”的研究论文,研究论文发现杆状病毒特异性破坏宿主细胞出核转运通路。
苜蓿银纹夜蛾核型多角体病毒(AcMNPV)在感染昆虫宿主细胞过程中,会将大量病毒蛋白质与宿主蛋白质转移到胞核,用于在胞核内完成病毒基因组复制、基因转录与新生核壳体装配等病毒复制过程。然而,AcMNPV如何诱导大量蛋白质发生核聚集的机制仍然未知。
CRM1是真核细胞内最重要的蛋白质出核转运通路。近日,通过构建AcMNPV瞬时表达文库,陈新文学科组筛选到AcMNPV晚期基因产物Ac34可以特异性关闭宿主细胞依赖CRM1的蛋白质出核转运通路,从而在病毒感染晚期,将与病毒复制相关的蛋白质,包括肌动蛋白聚合因子Arp2/3复合物等富集在胞核内,用于核壳体装配等重要过程。
相比过去发现的多种病毒破坏宿主细胞的蛋白质入核转运通路(主要是抑制干扰素的产生),该研究首次发现杆状病毒可以特异性破坏宿主细胞的出核转运通路,从而为病毒复制服务。
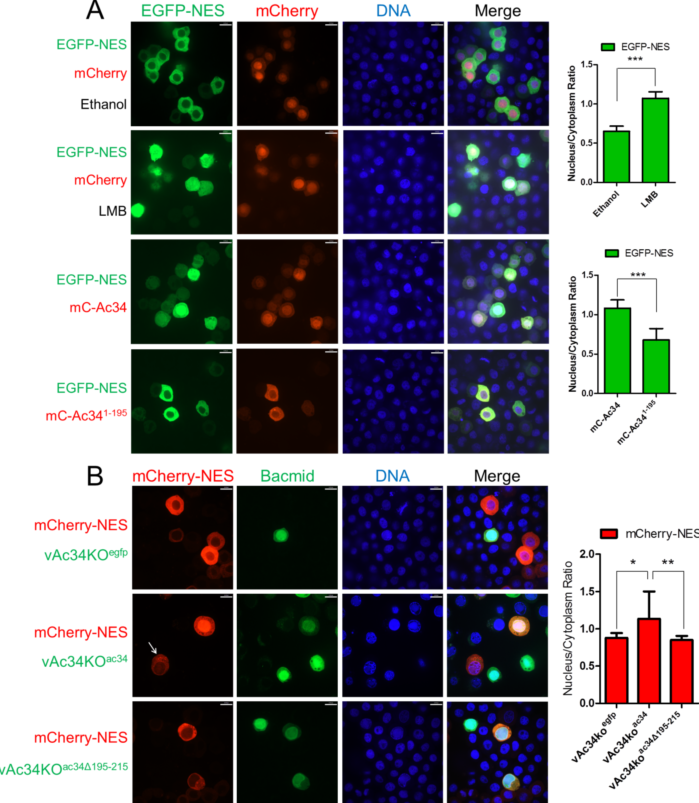
AcMNPV感染过程中,Ac34可以抑制CRM1依赖的出核通路
原文链接:
Autographa californica Multiple Nucleopolyhedrovirus Ac34 Protein Retains Cellular Actin-Related Protein 2/3 Complex in the Nucleus by Subversion of CRM1-Dependent Nuclear Export
原文摘要:
Actin, nucleation-promoting factors (NPFs), and the actin-related protein 2/3 complex (Arp2/3) are key elements of the cellular actin polymerization machinery. With nuclear actin polymerization implicated in ever-expanding biological processes and the discovery of the nuclear import mechanisms of actin and NPFs, determining Arp2/3 nucleo-cytoplasmic shuttling mechanism is important for understanding the function of nuclear actin. A unique feature of alphabaculovirus infection of insect cells is the robust nuclear accumulation of Arp2/3, which induces actin polymerization in the nucleus to assist in virus replication. We found that Ac34, a viral late gene product encoded by the alphabaculovirus Autographa californica multiple nucleopolyhedrovirus (AcMNPV), is involved in Arp2/3 nuclear accumulation during virus infection. Further assays revealed that the subcellular distribution of Arp2/3 under steady-state conditions is controlled by chromosomal maintenance 1 (CRM1)-dependent nuclear export. Upon AcMNPV infection, Ac34 inhibits CRM1 pathway and leads to Arp2/3 retention in the nucleus.
doi:10.1371/journal.ppat.1005994
作者:陈新文

